Investigators Pre-Screening (IPS)
Ensures the Best Match between a Targeted Indication, a group of Clinical Investigators, and the Patients to be Enrolled
GIVEN AN INDICATION...
Scope
- to identify as many potential investigators as possible, using Bouée databases, the Contractor databases, as well as other available sources,
- to survey investigators regarding:
- past clinical experience,
- current clinical activity,
- patients’ profiles & numbers for both the potential investigator and his institution,
- potential number of cases for the targeted indication,
- GCP awareness,
- GCP compliance for both the potential investigator and his institution,
- capability to conduct the envisioned clinical trial for both the potential investigator and his institution,
- supports needed by the potential investigator and / or his institution to carry out the envisioned clinical trial,
- willingness to participate to the clinical trial.
Pre-Screening Method
(upon reception of completed questionnaire, send gift of appreciation),
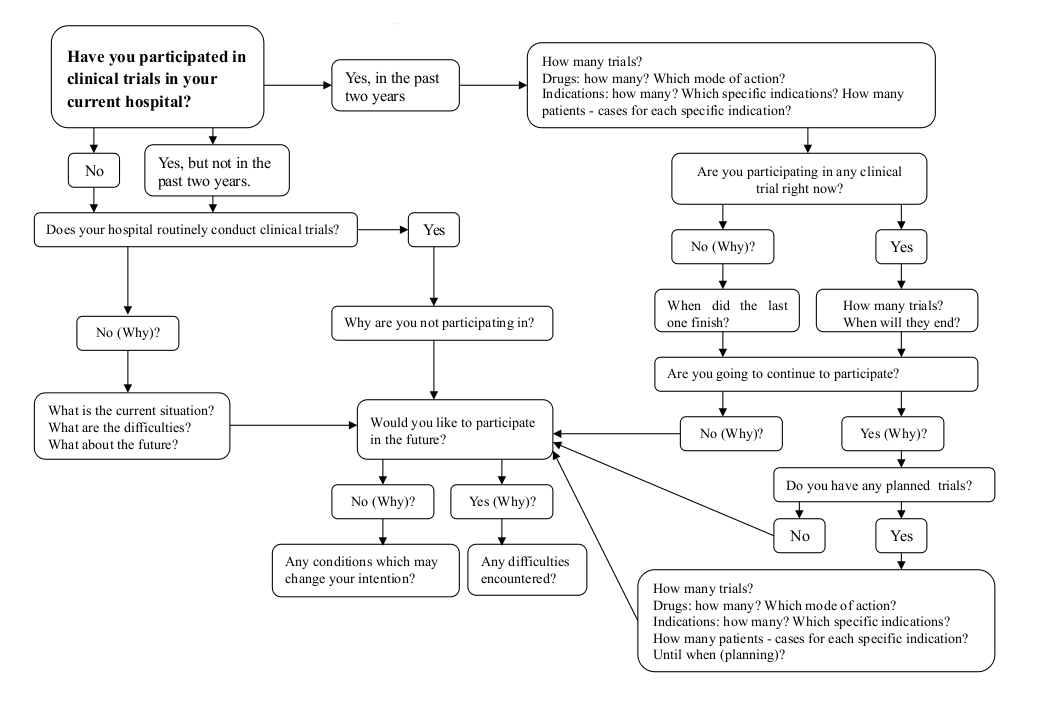
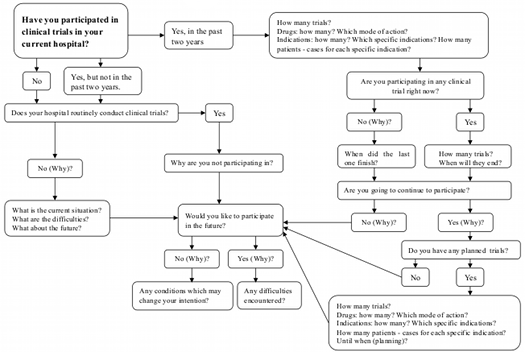
Questionnaire Workflow
![]()
(referring to the clinical trial synopsis provided by the Contractor)
If you were to participate in this clinical trial,
how many patients do you have?
(Yourself) How many a month at total?
How many can participate within a certain period (depends)?
(Hospital) How many a month at total?
How many can participate within a certain period (depends)?
BRI can also explore doctor's interest in / attitude towards:
- Clinical Research Coordinators (CRCs)
- Source Data Verification (SDV)
- Statistical Analysis
- Etc
Investigators’ & Contractor’s privacy rights: Options
- (up to the Contractor, but more often exercised in case of a non-competitive indication such as in an orphan drug development, etc.), the Contractor’s name is disclosed in the questionnaire, and BRI seeks the interviewee’s authorization to disclose his name to the Contractor. Subsequently, the analyzed data supplied by BRI to the Contractor bears the potential investigators names.
- (up to the Contractor, but more often exercised in case of a competitive indication, etc.), the Contractor’s name is not disclosed in the questionnaire. Subsequently, the analyzed data supplied by BRI to the Contractor doesn’t disclose the potential investigators names per se, only ID numbers. The Contractor selects which investigators to contact. In a follow-up procedure, BRI contacts the selected interviewees, discloses the Contractor’s name and seeks the interviewee’s authorization to disclose his name to the Contractor..
Note: until we have a mutually agreed and honored contract with our client or prospect on the concerned topic, our Documents, Research Proposals and the relevant Research Procedures are the sole property of Bouée Research Institute & L207. They cannot be used, directly or indirectly, partly or as a whole, without the prior written consent of an authorized person from Bouée Research Institute or L207.